Is the Flu Vaccine Safe for Toddlers?
A critical analysis of a study allegedly proving the safety of influenza vaccines for children
6 months to 23 months of age.
Citation -
"Safety of Trivalent Inactivated Influenza Vaccine in Children 6 to 23 Months Old",
10/25/06
JAMA. 2006;296:1990-1997.
Source URLs:
PDF |
Abstract |
Full Text
Abstract:
Despite the conclusion of the authors of the above study and subsequent JAMA article, the influenza vaccine was
NOT
proven safe for children under two years of age by the reference study.
[1]
The methodology of the study was to compare the number of Medically Attended Events in a group of children in a period
after vaccination versus two equal length of time "control" periods for the same group.
Study Flaws include the following:
- The first reference control period suffered from inflated figures due to population selection.
- The second reference control period was clearly a risk period which included many side effects due to the flu vaccine.
- The adverse medical events on the day of vaccination were rejected from the risk analysis data.
- The risk period chosen for study was the risk period with the fewest medical events in the month after vaccination.
- All risk periods were shortly after the date of vaccination and therefore could not detect delayed reactions to
mercury and other serious adverse effects caused by the vaccine.
Introduction:
Beginning in 2004 flu vaccine began to be recommended for all children between 6 and 23 months. There was very little
information available about the safety of flu vaccine in this age group. Even worse, the
Cochrane Collaboration,
an international organization for medical evaluation revealed that
"Two efficacy studies involving
about 1,000 toddlers indicate that flu shots containing inactivated virus — the only vaccine approved for this
age group — are
no more effective at preventing the flu than placebo."
Vaccine promoters have had to face the charges leveled against the flu vaccine's safety and efficacy.
The JAMA article released in late October claims to address the lack of safety data for the flu vaccine.
The JAMA article's significant claims are as follows:
- The medically attended events befoe and after the 69,391 vaccinations in 45,356 children were measured.
- Thirteen medically attended events were less likely to occur after flu vaccination in this study group.
- No diagnosis was significantly associated with flu vaccination.
- "This study provides additional evidence supporting the safety of universally immunizing
all children 6 to 23 months old with influenza vaccine."[1]
Scientific Principles Involved
How does one measure either the adverse effects or benefits of a vaccination?
First one has to define the quantity or quanitities desired to be measured. In this study, the desired quantity was the number
of acute (transient) medical events that
closely follow vaccination AND which are causally related to the vaccination. Because this figure can not be measured
directly, two other quantities will have to be measured first. The desired quantity will be the difference between
the two measurements. That is, the vaccine adverse effects will constitute an
excess of medical events following
vaccination over the
expected background of acute events determined by a control period before vaccination.
A
risk period is a period of time following vaccination during which medical events are counted
.
A
control period is a period of time during which medical events are counted but which are known to be
unrelated to the vaccination in question. The control gives a reference number of medical events which
can be used to predict the background number of medical events in the risk period that are not caused by the vaccination.
The
risk of a certain diagnosis of disease occurring after vaccination compared to before vaccination may be
listed as a ratio called a
Odds Ratio.
Medical
diagnosis with a
Odds Ratio of less than one means that the diagnosis was less likely to occur
after vaccination versus the
risk period.
Diagnosis with an
Odds Ratio of greater than one means that the diagnosis was more likely to occur after vaccination
in the
risk period.
I.E. An
Odds Ratio of .75 means that a certain diagnosis is only 75 percent as likely to occur in the risk period as
in the control period. If diagnosis of a certain disease occurred 100 times in the control period then a .75 risk means
only 75 diagnosis occurred in the risk period. Suggesting that the vaccine is not significantly related to this
risk and may even be protective.
Medical literature reflects that it is common for some symptoms like fever, cough, redness at vaccine
injection site, headaches and sore throat to be more likely to occur after vaccination, that is these incidents
have Odds Ratios greater than 1. It is also generally agreed upon, even in the pro-vaccine community, that there is ]
NO protective
value of flu vaccination for the flu in the two weeks following vaccination. Thus it is unscientific to suggest that
the vaccine had a protective effect which lasted only two weeks against 13 diagnosis.
Generally speaking, the number of medically attended events (MAE) in two periods, the control period and the risk period,
determine
the conclusions for a study. Thus, it is very important to carefully chose the periods and also to do accurate counts of the
medically attended events in these two periods. We will see for the JAMA study there there were several risk periods, a
common practice,
and two control periods, an uncommon practice and clearly incorrect in this case because the second control period was
admittedly a risk period.
Problems/Irregularities
An old saying holds that,
"Figures do not lie, but liars do figure."
How well did our JAMA study apply the principles we have outlined above?
Let's have you answer that above question by answering a couple more questions
(1) Question: Given the data of the following risk periods, which do you call the Primary Risk Period and the focus of your
study.
1. Vaccination Day zero (0) with its high number of medical events, a fact which is admitted but for which the number is unpublished.
2. Days 1-15 after vaccination with approximately 5095 medical events.
3. Days 15-28 after vaccination with approximately 6449 medical events.
(1) Answer: Hold out your hands. If the first thing that comes into your hands is a child, then use the data from all three periods
or pick period 3 above for your Primary Risk Period. If the first thing that comes into your hands is a check from
Big Pharma, then pick number 2, the study authors chose number 2 and neglected 1 and 3.
(2) Question: Given the data of the two following periods, which do you pick for your Control Period(s).
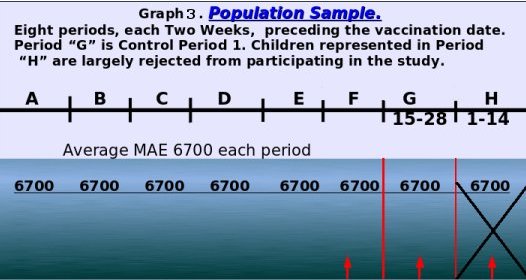
1. The period of 14 days before vaccination in which children were rejected due to medical events creating a deflated MAE.
2. The period of 14 days in the 15-28 day period before vaccination which, due to population selection, MAE is inflated.
3. The period of 15-28 days after vaccination which has many more medical events than the 1-14 period after vaccination.
(2) Answer: If you are scientifically minded, then you recognize than none of these periods are suitable as a
Control Period and you will do the
work necessary to generate accurate numbers for a genuine Control Period. On the other hand, in the reference study,
the period just before vaccination
was rejected for use, and both periods 2 and 3 were used to "predict" the baseline number of medical events in the
Primary Risk Period.
It is proper to reject the period just before vaccination due to its artificially low number of MAE,
a condition which is created by rejecting for vaccination any child who was sick in this period.
The period 15-28 days after vaccination is not a proper control period but instead is a risk period. We will refer to this
as the Secondary Risk Period hereafter for clarity.
The period 15-28 days before vaccination does not contain an accurate count for MAE as shown below in bias sections
2 and 3.
If you have not already done so, it may be best to read the reference
JAMA article before trying to digest
my comments.
Some quotes from the JAMA article follow which sum the JAMA authors' conclusions with some added emphasis:
Design, Setting, and Participants
Retrospective cohort using self-control analysis, with chart
review of significant medically attended events at 8 managed care organizations in the
United States that comprise the Vaccine Safety Datalink. Participants were all children in the
Vaccine Safety Datalink cohort 6 to 23 months old who received trivalent
inactivated influenza vaccine between January 1, 1991, and May 31, 2003 (45,356 children with 69,359 vaccinations)."
Results: "... only 1 diagnosis,
gastritis/duodenitis, was more likely to occur in the 14 days after trivalent inactivated influenza vaccine."
"Thirteen medically attended events were less likely to occur after trivalent inactivated influenza vaccine,
including acute upper respiratory tract infection, asthma, bronchiolitis, and otitis media."
Did you notice the statement that
"Thirteen medically attended events were less likely to occur after
trivalent inactivated influenza vaccine,
including acute upper respiratory tract infection, asthma, bronchiolitis, and otitis media."? [1]
Question: What is the statement, "less likely to occur", based upon?
Answer: Two control periods each had a higher number of medically attended events (MAE) than did
the 14 day period which started 24 hours after vaccination.
It should be obvious that the above conclusion is suspicious, to say the
least, and calls for a careful examination of the validity of both control periods as well as the correctness of the
number of events in the Primary Risk Period.
Question: Note the statement in the above quotes, "Results: "... only 1 diagnosis,
gastritis/duodenitis, was more likely to occur in the 14 days after trivalent inactivated influenza vaccine."
What does this mean?
Answer: That is a really good question. In the Jama article, in Table 2, eleven diagnosis
are listed as follows:
Column marked R/C1 is the Primary Risk Period to Control Period 1 Ratio.
Column marked [R2/C1] is the secondary Risk Period compared to Control Period 1.
ED = Emergency Department
Secondary Risk Period is labeled in the JAMA study as Control Period 2.
Diagnosis R/C1 [R2/C1]
Anemia 10/3 [8/3]
Convulsions (ED) 33/15 [27/15]
Gastritis/duodenitis 11/2 [3/2]
General Symptoms (ED) 6/2 [4/2]
Lymphadentis 13/3 [12/3]
Noninfectious gastroentertis (ED) 36/18 [54/18]
Noninfectious gastroentertis (Inpatient) 19/9 [23/9]
Serum reaction 5/2 [2/2]
Sickle cell anemia 9/6 [6/1]
Urticaria (rash) 9/4 [35/4]
Viral enteritis 4/7 [2/7]
Of the 11 diagnosis above, 10 had less incidence in Control Period 1 than occurred in either the Primary Risk Period or the
Secondary Risk Period.
Let us look at a two important statements from the JAMA article,
"Of note, the 3-day risk window in the outpatient setting included days 1 to 3, because inclusion of day 0 (the day
of vaccination) has been shown to result in spurious signals when using self-control methods with outpatient data."
[This time period was included in analysis of ED and inpatient settings.] In other words, there are a high number of
MAE on the day of vaccination leading people to correctly believe that the vaccine is harmful.
the "healthy vaccinee" effect
"Because physicians tend to administer vaccines to healthy children, medically attended event incidence rates
in the 1 to 14 days before vaccination may underestimate true background rates
(the "healthy vaccinee" effect).27 We therefore excluded days 1 to 14 prior to vaccination from the analysis."
In plain language, using the two week period prior to vaccination with its deflated MAE rate as a control period
tends to lead one to
conclude that the vaccine is harmful. We will discuss the effect of the "healthy vaccinee" effect upon MAE rate
calculations later, as well as the fact that
using the third and fourth week period prior to vaccination with its inflated MAE rate as a control period
tends to lead one to the false conclusions that the vaccine is beneficial and safe.
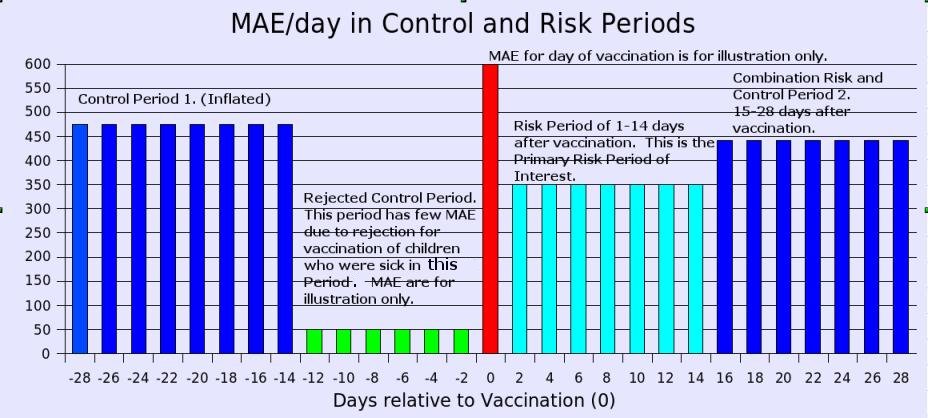
Time line:
Prior Vaccination Control 1 Rejected VAX Risk Control/Risk
???? | 15-28 | 1-14 | 0 | 1-14 | 15-28 |
Risk periods:
0 to 2 days post vaccination
1 to 3 days post vaccination
1 to 14 days post vaccination (primary period of interest)
15-42 days post vaccination (secondary period of interest)
1-42 days post vaccination
Control period 1: 15-28 days prior to vaccination.
Control period 2: 15-28 days after vaccination. Remember, we are labeling this as Secondary Risk Period.
Let's Debunk the validity of Control Period 2 first:
Control period 2: 15-28 days after vaccination:
Hmmm, that's clever, using an
undisputed risk period, 15-28 days after vaccination to constitute a
"control period" for predicting the number of MAE in the
early risk period of 1-14 days after vaccination.
It may be clever to use a control period within the longer risk period, but scientifically valid it is
NOT!
At least, it is not valid for calculating what the MAE rate in the
first major risk period should be.
Until you actually measure the number of MAE in these
two periods you can not predict if the number of
MAE in the period immediately following vaccination either should or will be less or more than the number of MAE in the
next risk period two weeks later.
If the MAE incidence in this Secondary Risk Period had been
lower than the Primary Risk period, would it be a
valid control period for the purpose of estimating MAE incidence in the Primary Risk period?
NO, of course not. Such an occurrence would simply tell us the obvious, that vaccine adverse effects were greater
in the Primary Risk Period. However, in the JAMA study, the MAE incidence was
higher in the Secondary Risk Period versus
the Primary Risk Period, and
this likewise does not allow us to calculate the extent of vaccine adverse side effects in the Primary Risk Period.
However, the
fact that these risk periods close to the vaccination date had differing MAE rates only
tell us
which risk period had the most vaccine side effects but without a valid control it can not be known
what the magnitude of side effects alone are in either period.
Actually, many people
would probably guess that the number of MAE in the two weeks immediately following vaccination will be higher
than in the next following two weeks.
However, this was not the case in this study.
What does this tell us?
Was the vaccine protective for the first two weeks following vaccination?
Or, did the symptoms of vaccine harm increase 3 and 4 weeks following vaccination versus the first two weeks?
Only a few people will buy that a vaccine is "protective" for the first two weeks following vaccination after which the
"protection" immediately falls to near zero. In the first two week period following vaccination children commonly
experience more fevers and even
Influenza Like Illness (ILI). Chronic illness due to vaccination takes longer to emerge.
Given that the flu vaccine administered in the years
covered by this study
contained mercury, a substance difficult to excrete for some children, and also contained aluminum a substance bonded to the vaccines'
antigens for the purpose of making the antigens difficult to eliminate by all individuals, it perhaps should come
as no surprise that the number of MAE should rise in the second two week period after vaccination.
We conclude that the harm of the vaccine
does not end at 2 weeks but continues to increase in strength.
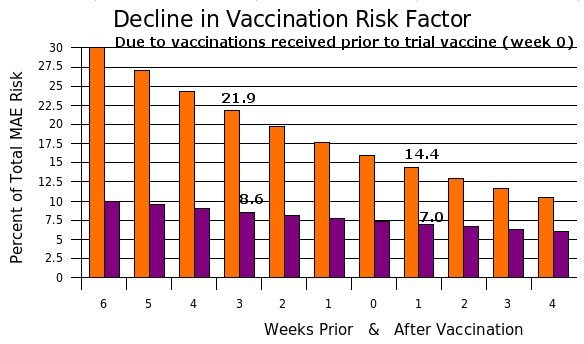
Debunking the published MAE rates for Control Period 1
For a clear picture of the published, but uncorrected and thus incorrect, MAE rates in the Control Period 1
as well as the risk periods see graph 1 immediately below and
for some of the actual numbers of MAE incidence see table 1 lower on this page.